Making immunotherapy more effective for breast cancer
Anushka-profile-pic-resize.jpg

Anushka Dongre, assistant professor in the Department of Biomedical Sciences and an adjunct assistant professor in the Department of Microbiology and Immunology, focuses on the intersection of cancer biology and immunology.
By all accounts, Dr. Anushka Dongre sits at the cutting edge of cancer science. As assistant professor in the Department of Biomedical Sciences and an adjunct assistant professor in the Department of Microbiology and Immunology, she focuses on the intersection of cancer biology and immunology, garnering multiple grants and awards, including most recently the Affinito-Stewart award from the President’s council of Cornell Women and the Breast Cancer Research Faculty Grant from the Breast Cancer Coalition of Rochester. She’s published several papers on the biology of cancer immunotherapy — including a recent review article in Molecular Diagnosis & Therapy on the use of immune checkpoint inhibitors in the treatment of breast cancer.
But it wasn’t always this way. While immunotherapy has become a well-known term in the cancer biology field, it was only a few years ago that the idea was viewed as new-fangled. “People were talking about it, but they hadn’t realized the full potential of the technology,” says Dongre.
And, Dongre was a bit of an interloper — as a Ph.D. student in immunology at the University of Massachusetts-Amherst, she became fascinated with cancer biology, going on to join the lab of Dr. Robert Weinberg, a pioneering cancer researcher at the Whitehead Institute in MIT. Initially, she struggled to get her post-doctoral grants funded.
This all changed in 2018, when Dr. James P. Allison and Dr. Tasuku Honjo received the Nobel Prize in medicine for their groundbreaking work in immune checkpoint blockade therapy. Their work uncovered ways to harness the body’s own immune system to fight cancer, leading to the development of several successful drug treatments.
Suddenly, Dongre was on the right side of the cutting edge, leading her to take a position at Cornell, where her lab primarily studies how the plasticity of breast cancer cells drives immunotherapy-drug-resistance.
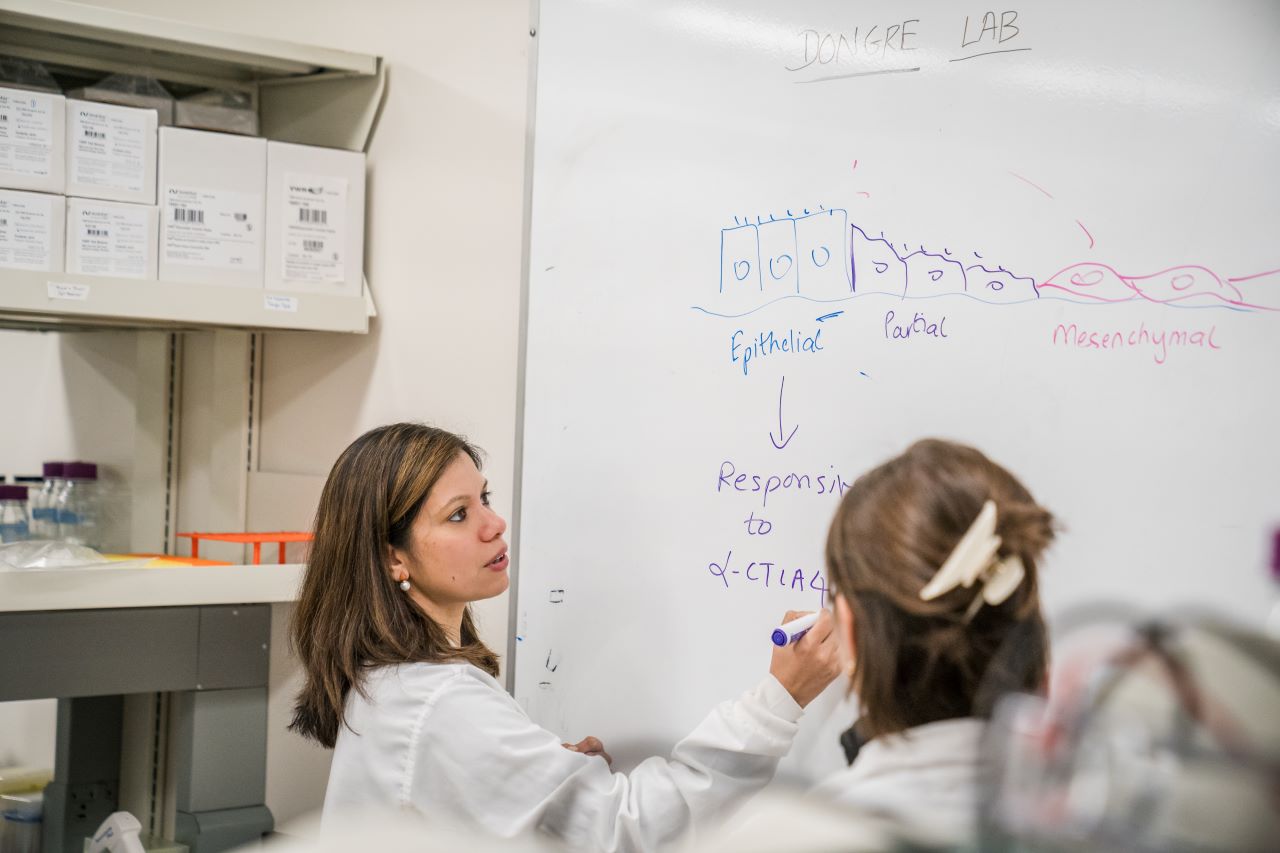
Immunotherapy
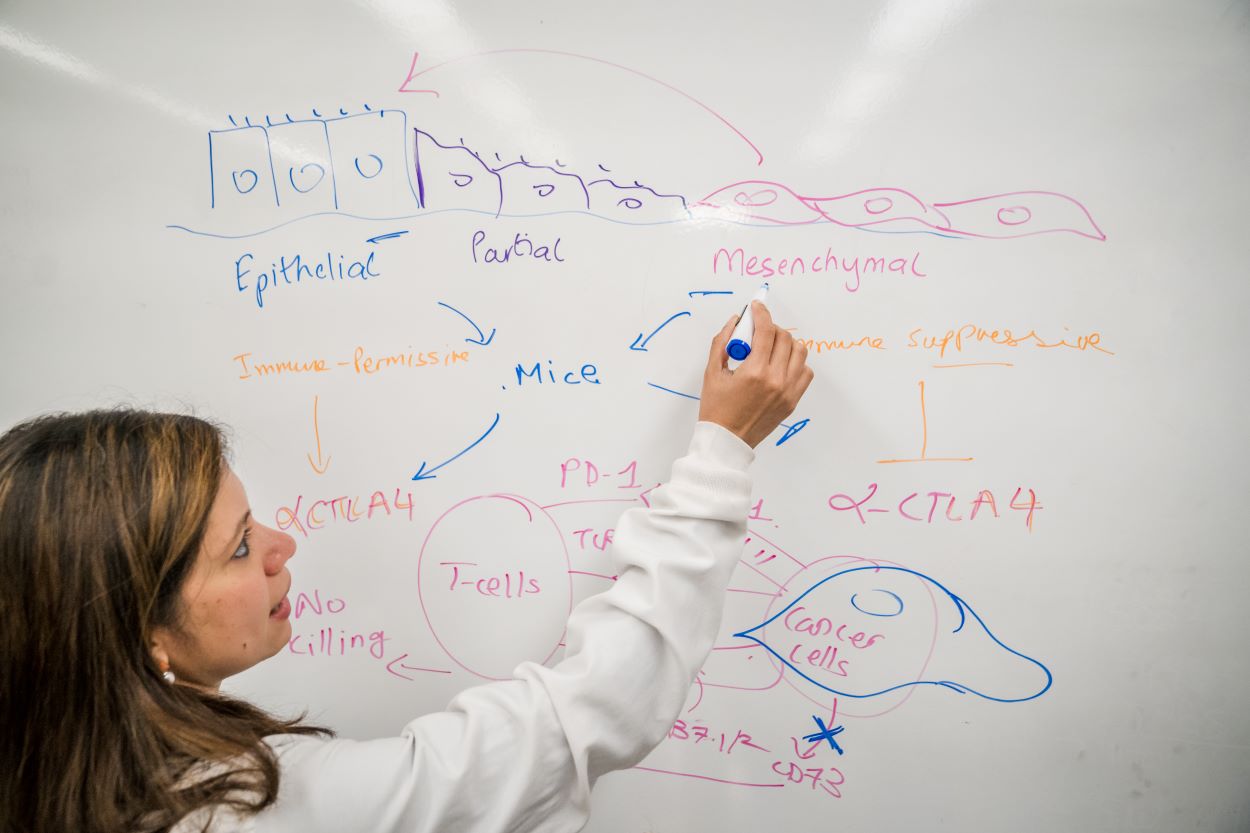
Immunotherapy works by freeing up the body’s own natural defense systems to battle cancer cells. Typically, cancer cells can evade the body’s immune system by binding T-cell receptors, effectively turning off their attack mode. Immunotherapy drugs block these receptors, thereby empowering the T-cells to destroy the cancer cells. Key receptor drug targets include receptors called PD-1 and CTLA4. Anti PD-1 and CTLA4 drugs have been shown to work well in restoring T-cell function, yielding effective treatments for some lung and skin cancers. However, breast cancer has only a 30% response rate to immunotherapy, leading to Dongre’s driving question — why do some cancers respond beautifully to immunotherapy treatments, while others seem to find ways to fight it?
A dangerous transformation
Dongre suspects epithelial-mesenchymal transition, or EMT, is the culprit. EMT occurs when epithelial cells — those found in the lining of various internal body surfaces —transform into mesenchymal cells which can detach and travel around the body, and are integral to wound healing. However, cancer hijacks this process for its own gain. In breast cancer tumors, when EMT occurs, epithelial cells transform into mesenchymal cells, which can initiate metastasis.
Dongre was the first to surmise that EMT might have an impact on cells’ immune responses. “I was intrigued by how mesenchymal cancer cells secreted TGF-B1,” she says, referring to the cytokine that plays a key role in cell proliferation, differentiation and apoptosis. The cytokine helped to reinforce a mesenchymal state in cancer cells. “As a trained immunologist, I hypothesized that these mesenchymal tumors may therefore be immunosuppressive,” Dongre says. This idea had never been addressed in breast cancer due to the lack of tumor models in which to test the idea. So, Dongre took it upon herself to create the models herself.

Malignant minority
Using these models, Dongre went on to show in a Cancer Research paper published in 2017 that EMT has real impact on cancer cells in vivo. Dongre’s novel breast cancer murine models featured cell lines that either expressed more epithelial or more mesenchymal features. When these cells were implanted into mice, the mesenchymal tumors were much more resistant to immunotherapy drugs compared to the epithelial tumors. “We found that, not only do mesenchymal carcinoma cells recruit immunosuppressive cells to their tumors, but a small minority of more-mesenchymal cells actually protect the majority of their epithelial neighbors within such tumors from an immune attack,” says Dongre. “This is clinically very important, since most carcinomas treated in the oncology clinic have a small proportion of more-mesenchymal cells that may well be protecting the tumor as a whole from immune attack. As long as you have a few mesenchymal cells, the tumor won’t respond.”
Eliminating mesenchymal cells
But what, exactly, was the secret to the mesenchymal cells’ defense system? In research published in a 2021 issue of Cancer Discovery, Dongre looked for the source of that resistance, and found that an enzyme known as CD73 seemed to be the key. When Dongre removed the enzyme, mesenchymal cells suddenly became susceptible to cancer drugs, particularly a class of immunotherapy drugs known as “anti-CTLA4”, which enhance immune responses.
“This could mean that treatments that target CD73 could be particularly beneficial for breast tumors that display EMT tendencies,” says Dongre. The work also showed for the very first time that mesenchymal cancer cells can be completely eliminated by tweaking their immuno-modulatory properties. “The EMT program promotes metastasis and drives resistance to multiple therapies,” Dongre says. “So, our findings can change cancer treatment by eliminating these mesenchymal cells altogether. We are one of the first few folks to demonstrate this.”
This research has led to her becoming the 2023 Breast Cancer Research Faculty Grant recipient from the Breast Cancer Coalition of Rochester. The $50,000 award goes specifically towards Dongre’s investigation of CD73 as a possible therapeutic target.
Overall, Dongre hopes to see her discoveries make their way into clinical decisions. Right now, a tumor’s EMT status is not currently part of the clinical predictions of how well immunotherapy will work for a patient. “People aren’t looking at EMT as a predictor for immunotherapy success,” she says. “In the future, the hope is that we could incorporate it into treatment decisions and use immunotherapy to eliminate more-mesenchymal cancer cells.”
Looking ahead
Dongre has several exciting projects on the horizon. She plans to further investigate how just a few mesenchymal cells manage to protect an entire tumor of their fellow epithelial cells. She’ll also dive deeper into why anti-CTLA4 drugs seem to have the most potential for killing mesenchymal cancer cells, and develop a variety of in vivo models to test whether the EMT program directly results in immunosuppression at the site of metastasis.
And, she’s not stopping at ways to defend against EMT — she’s looking for ways to reverse it altogether, by turning mesenchymal cells into epithelial cells instead. “We want to see if we can take back control of that process — if that might be a different way of enhancing susceptibility to anti-tumor immunity,” says Dongre.
Written by Lauren Cahoon Roberts
Photos by Darcy Rose




