Todhunter Laboratory
The Todhunter laboratory has been subsumed by the Cornell Veterinary Biobank (CVB). All the hip dysplasia genetic samples are now housed in the CVB. The broad sweep of orthopedic genetic research is still the focus of Dr. Todhunter’s research. The focus has always been on prevention as opposed to treatment of hip dysplasia and other inherited orthopedic traits in dogs. We try to identify genetic markers (single nucleotide polymorphisms) that point to the associated or causal genes and the genes themselves underlying the development of canine hip dysplasia and secondary hip osteoarthritis. We have begun genetic mapping of rupture of the cranial cruciate ligament and elbow dysplasia. However, it takes time and resources to discover genes underlying complex traits and diseases in any species.
What is the Cornell Veterinary Biobank?
The CVB was initiated at Cornell in early 2006 to store genetic material from any animal admitted to our Hospital with known or presumed disease of genetic origin. Strong collaboration with all Hospital services and the Hospital staff has enabled this archive to thrive. The archived samples along with accurate phenotypes are used to discover the genetic basis of disease. As of mid 2014, the CVB contains 11,000 DNA samples (from well-phenotyped animals). The accompanying pie chart summarizes the most common diagnostic categories in the Biobank as of mid-2014. Behavioral, dietary and environmental survey data is stored with currently archived samples. The most common breed of dog represented in the Biobank is the semi-domesticated village dog ( in collaboration with the Boyko lab in the Dept. of Biomedical Sciences) followed by the Labrador Retriever, Golden Retriever, Boxer, English Springer Spaniel, German Shepherd, mixed breed, Wire-haired Pointing Griffon, Rottweiler, and Border Collie.
Research
In the Cornell University Hospital for Animals (CUHA) we evaluated healthy aged Labrador Retrievers as controls for genetic mapping studies. Health screening includes a complete blood count and a chemistry panel; urinalysis; general physical examination, orthopedic, ophthalmologic, oncology, neurologic and cardiac exams; a nutrition consult (for consistent body condition score), hip radiographs, serial force plate gait analysis, body measurements for morphometric studies, and upper airway examination, all conducted by board certified specialists. With this panel, we can identify, or rule out, multiple diseases inherited in Labradors, such as hip dysplasia, ruptured anterior (cranial) cruciate ligament, mast cell tumor, T and B-cell lymphoma, posterior polar cataracts, liver disease, laryngeal paralysis, degenerative myelopathy, epilepsy, tricuspid valve dysplasia and obesity. One hundred and forty Labrador Retrievers have been screened to exclude these diseases or traits. We will continue accurate phenotyping of aged dogs of other pure breeds to be used as common controls for mapping studies of multiple disorders.
Using 4,200 Illumina High Density mapping arrays, dogs with the following traits and diseases have been genotyped in collaboration with the Boyko lab and Zoetis Inc. in order to discover genomic regions harboring mutations causal for these disorders: owner-directed aggression; hip dysplasia; elbow dysplasia; ruptured cranial cruciate ligament; lymphoma, dilated cardiomyopathy, idiopathic epilepsy and primary ciliary dyskinesia in Wolfhounds; endochondral ossification and growth rate related the hip dysplasia, protein-losing enteropathy, myxomatous mitral valve degeneration, osteosarcoma, lymphoma, hemangiosarcoma, granulomatous colitis, and mast cell tumor.
Personnel Supported in the Biobank
 Marta Castelhano, DVM is the Director of the CVB. Her responsibilities include overseeing the sample collection efforts (including tissue collection in collaboration with our surgery and Community Practice Services) and phenotypic ascertainment of each sample banked. Dr. Castelhano is the lead investigator of the Labrador retriever mast cell tumor genetic mapping study, a collaborative effort involving small animal Oncology faculty (Drs. Balkman and Hume) at the Cornell University Hospital for Animals (CUHA), the CVB, and 16 Oncology centers/University hospitals across the United States. Dr. Castelhano is a collaborator in many other mapping projects with internal and external investigators (Drs. Todhunter, Sutter, Boyko, Simpson, Ainsworth, Casal, Moise, and Henthorn). She also staffs our Pediatrics and Medical Genetics service with adjunct faculty Magi Casal, University of Pennsylvania. This service offers consultations to other specialty services and outside hospitals/rDVMs. Dr. Castelhano also mentors DVM students who assist with genetic mapping projects in the CVB.
Marta Castelhano, DVM is the Director of the CVB. Her responsibilities include overseeing the sample collection efforts (including tissue collection in collaboration with our surgery and Community Practice Services) and phenotypic ascertainment of each sample banked. Dr. Castelhano is the lead investigator of the Labrador retriever mast cell tumor genetic mapping study, a collaborative effort involving small animal Oncology faculty (Drs. Balkman and Hume) at the Cornell University Hospital for Animals (CUHA), the CVB, and 16 Oncology centers/University hospitals across the United States. Dr. Castelhano is a collaborator in many other mapping projects with internal and external investigators (Drs. Todhunter, Sutter, Boyko, Simpson, Ainsworth, Casal, Moise, and Henthorn). She also staffs our Pediatrics and Medical Genetics service with adjunct faculty Magi Casal, University of Pennsylvania. This service offers consultations to other specialty services and outside hospitals/rDVMs. Dr. Castelhano also mentors DVM students who assist with genetic mapping projects in the CVB.
 Liz Corey, BS is a specialist research technician who currently oversees the laboratory involved with the CVB. She is responsible for extracting, quantifying, and archiving DNA and RNA, maintaining inventory in the DNA bank database, and preparing and shipping samples to collaborators. She is developing the archive to include tissue collections, including helping to develop new SOPs for sample collection. Liz routinely discusses sample processing with CUHA clinicians and outside collaborators and suggests blood and tissue handling methods to optimize yields and quality for unique field collections. She prepares DNA plates for genotyping in collaboration with the Dr. Schweitzer in the Sequencing Center of the Cornell Life Sciences Core Laboratories. She provides instruction to students, other technical staff, and faculty on protocols, laboratory safety practices and waste disposal.
Liz Corey, BS is a specialist research technician who currently oversees the laboratory involved with the CVB. She is responsible for extracting, quantifying, and archiving DNA and RNA, maintaining inventory in the DNA bank database, and preparing and shipping samples to collaborators. She is developing the archive to include tissue collections, including helping to develop new SOPs for sample collection. Liz routinely discusses sample processing with CUHA clinicians and outside collaborators and suggests blood and tissue handling methods to optimize yields and quality for unique field collections. She prepares DNA plates for genotyping in collaboration with the Dr. Schweitzer in the Sequencing Center of the Cornell Life Sciences Core Laboratories. She provides instruction to students, other technical staff, and faculty on protocols, laboratory safety practices and waste disposal.
 Susan Garrison, LVT is a medical genetics technician whose duties include selecting cases for DNA and tissue banking and obtaining client consent for blood and tissue archiving during appointments in the Cornell university Hospital for Animals. She collects DNA (blood draw) or relevant tissue samples at surgical biopsy time or at definitive surgical correction, and processes the sample (prepares for RNA extraction, flash freeze or cell culture). She also assists in the performance of necropsies to obtain tissue for genetic studies.
Susan Garrison, LVT is a medical genetics technician whose duties include selecting cases for DNA and tissue banking and obtaining client consent for blood and tissue archiving during appointments in the Cornell university Hospital for Animals. She collects DNA (blood draw) or relevant tissue samples at surgical biopsy time or at definitive surgical correction, and processes the sample (prepares for RNA extraction, flash freeze or cell culture). She also assists in the performance of necropsies to obtain tissue for genetic studies.
As part of the clinical service, Susan assists with patient appointments and exams in CUHA, providing genetic testing service and the genetic screening of aged pure breed controls. Susan is trained to answer routine medical genetics questions from clients and students (genetic tests offered, sample submission protocols, results and interpretation).
 Julie Jordan, LVT is also a research technician.
Julie Jordan, LVT is also a research technician.
Past and Current Funding
The CVB and genetic research it has spawned has been supported, accepted, and encouraged by the Cornell veterinary community. Genetic medicine is the burgeoning underpinning of every specialty. The original 3 years of College financial support for the Biobank lead to acquisition of an NIH 4-year R24 in 2008 ($225,000 direct costs each year for 4 years) and an ARRA supplement ($111,905 direct costs for 1 year) in 2010. This community resource provided a unique platform for philanthropy in support of genomics in the College partly resulting in the largest ever gift to the Veterinary College, in support of cancer and genomics. The Dean of the College also supports the infrastructure of the Biobank.
The Medical Genetics and Pediatric Service
The nascent Medical Genetics and Pediatric Service operates in parallel with the Biobanking effort of genetic archiving, mapping, and mutation discovery. This Service has its home in the Community Practice Service and includes Dr. Marta Castelhano, Dr. Magi Casal, Associate Professor of Medical Genetics, Pediatrics, and Theriogenology at the University of Pennsylvania and adjunct Professor at Cornell, and licensed veterinary technician Susan Garrison. Dr. Todhunter helps on the Service as needed for orthopedic screening. The Service will have an important role in the release of complex trait genetic marker tests orthopedic traits like hip dysplasia (also called genomic prediction). This Service teaches DVM students as part of consultation with other services and when individual dogs and cats are admitted for examination to rule in or out genetic traits and diseases. This service also offers medical genetic and pediatric rounds in the clinic. This service provides information about genetic tests offered in-house and those available externally. We offer an outreach service to rDVMs through phone consultations. In-house molecular genetic testing is done in partnership with the AHDC.
Canine Hip Dysplasia
Hip dysplasia is the most common complex orthopedic trait in medium and large breed dogs with incidences ranging from less than 10 to over 70% across pure breeds [offa.org for rankings based on Orthopedic Foundation for Animals (OFA) hip scores]. The clinically debilitating secondary osteoarthritis is also scored on the same radiographs used to measure hip conformation. Our analysis of 700,000 hip and 150,000 elbow records in the publically available OFA database demonstrate clearly that efforts to reduce the frequency and severity of hip dysplasia, and its secondary osteoarthritis in the USA based on radiographic evaluation (phenotype), have met with meager improvement in genetic quality as measured by estimated hip score breeding values since 1970 (Hou et al., 2014).
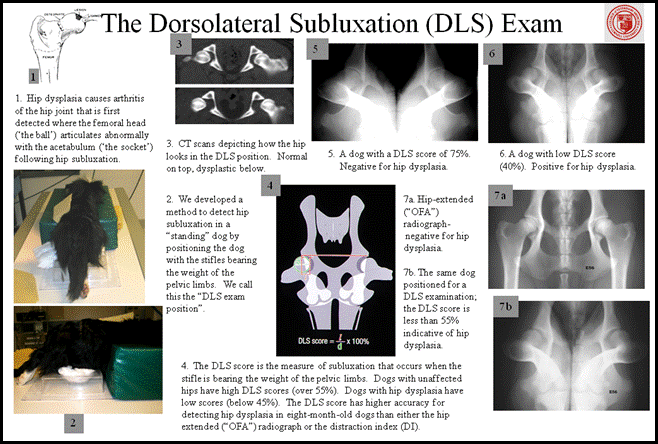
The diagnosis of hip dysplasia is confirmed radiographically but initially made clinically. Collected on the ventrodorsal extended-hip radiograph, the Norberg angle ranges from 70 degrees (worst) to 120 degrees and the Orthopedic Foundation for Animals hip score ranges on a 7-point scale from excellent hip conformation to severe hip dysplasia with secondary osteoarthritis. Maximum hip laxity (PennHIP™) is measured as the distraction index which ranges from 0.1 (tightest and best hip) to 1.2 (most lax hip). The dorsolateral subluxation test (developed by our group at Cornell) results in a score ranging from 20% (poorest hips) to 80% (best hips). Heritabilities of these hip traits vary by pedigree and across individual traits and range from ~0.2 to 0.6. Strong genetic correlations exist between each individual hip trait (Zhang et al., 2009). Based on our (Todhunter et al., 2005; Zhu et al., 2008; Mateescu et al., 2008; Phavaphutanon et al., 2009; Zhou et al., 2010) and others (Chase et al., 2005; Marschall and Distl, 2007, 2012) research, between 10 and 20 quantitative trait loci (QTL) likely control hip dysplasia expression.
THE DORSOLATERAL SUBLUXATION TEST AND CANINE HIP DYSPLASIA DIAGNOSIS
In an attempt to decrease the incidence of HD, screening of breeding animals for this trait is commonly performed and databases have been established to collate and distribute this data (Orthopedic Foundation for Animals, Fédération Cynologique Internationale, British Veterinary Association, PennHIP™). Screening is performed using radiography or, more rarely, computed tomographic or magnetic resonance scans. The most frequently performed screening technique is based on the ventro-dorsal extended hip radiograph (EHR). However, some databases require animals to be at least 1 year of age before the study is performed, and others require animals to be significantly older than this like the Orthopedic Foundation for Animals (2 years of age or older) before official certification. Often animals have been used for breeding before they reach this age leading to the perpetuation of dysplastic lineages. Several techniques have been developed to allow earlier diagnosis of HD such as the PennHIP distraction index, the dorsolateral subluxation score (DLS), and the Flückiger technique.
None of these techniques however provide a simple, rapid method for general practitioners to reliably screen dogs for unaffected hip joints or for hip dysplasia. These techniques require the use of circle gauges to derive the indices used to quantify the severity of joint abnormality of HD and the PennHIP and Flückiger techniques require the animal to be held manually during radiographic exposure and so cannot be performed in some countries due to radiation safety rules.
The Dorsolateral Subluxation Test
Some young dogs (8-12 months of age) with “good” hips when evaluated in the OFA style extended-hip radiograph, have positive Ortolani signs meaning that their hips are unstable. In my opinion, those dogs are susceptible to subsequent development of hip osteoarthritis and, by definition, dysplastic susceptible. A method was developed in the mid 1990’s that imaged the hips of dogs in a weight-bearing position i.e. how they would be positioned when walking. The diagram above illustrates and describes how the DLS test is accomplished. A plastic holder for the stifles that rest on the radiographic table is shown in the figure. Roll cotton is used to provide additional support for the limbs. The DLS score was shown to be the most accurate single technique for the detection of HD at 8 months of age. At 8 months of age, the sensitivity and specificity of the OFA-like radiograph was 38 and 96%, respectively and for the distraction index (values of >0.7 considered abnormal) were 50 and 89%, respectively and for the dorsolateral subluxation score (scores <55% were considered abnormal) was 83 and 84%, respectively. Another advantage for the DLS method is that it does not require manual restraint during radiographic exposure and has been shown to be reliable under both general anesthesia and sedation.
The S Measurement
We recently developed a method to simplify the DLS score calculation; a simple linear measurement (the S measurement) on radiographs for subluxation of femoral heads to be applied to the assessment of both unaffected and dysplastic joints. The figure below illustrates the S measurement (a direct measurement of the arrowed white line between the femoral head and the acetabulum indicated by the black arrows).
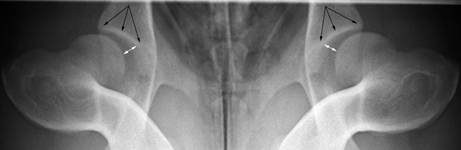
The S measurement is a reliable measure that correlates well to the DLS score: it is a simple, rapid method for general practitioners to screen dogs for unaffected hip joints. Magnification serendipidously had a desirable affect as it made abnormal hip joints look proportionally more abnormal relative to normal joints. Considering the overlap between dogs with and without hip dysplasia and the limits of agreement, we recommend using an S measurement cut-point of less than 5 mm to identify dogs that are highly unlikely to have HD. Using this cut-point, false-positive results are anticipated, which is desirable when the attempt is to cull dogs with HD. However, a radiographic phenotype cannot be used to eliminate HD from a population as it contains no information about the genotype of the dog. For effective breeding practices, estimated breeding values should be applied.
Radiographs made while dogs are help in this DLS position can be readily measured by veterinarians or sent to Cornell Veterinary College (care of Dr. Todhunter) for measurement. A report is generated which provides the DLS score and S measurement of each dog and its score relative to others in the breed. For dogs examined at Cornell, a standard OFA style ventrodorsal radiograph with the hips in the extended position would also be taken to provide the best estimate of the presence of secondary arthritis and for measurement of the Norberg angle. To aid in ongoing development of genetic tests for hip dysplasia, we request that an EDTA blood sample accompany the images.
Breeding Dogs with Good Genetic Potential
For Estimated Breeding Values (EBVs) for Canine Hip and Elbow Dysplasia, see link to Canine Hip Dysplasia Breeding Value web site. This is a searchable website that requires a dog's registration number or OFA number, and it must be publicly available in the OFA registry.
Genomic Prediction or Selection
Complex traits plague the health and longevity of pure breed dogs. Although the genetic control of Mendelian traits has been relatively easy to dissect, geneticists are slowly understanding to how to uncover the mutations for complex diseases. For fixed complex traits like breed body size or height, geneticists are meeting the challenge well. However, for commonly-segregating, complex traits and diseases the current challenge is how to use genome wide genotype information in a practical way until the mutations in linkage disequilibrium with the markers can be identified by sequencing. One way to employ the information in the association between the genotypes and the phenotype is to assess the combined genetic marker contribution to the trait independently of mutation discovery. Such a strategy is commonly referred to as genomic selection or prediction. It relies on the assumption that the genetic markers in close proximity to causal or contributing mutations segregate with the trait loci; they are in linkage disequilibrium. Thus the effect of the marker genotype on the trait is a reflection of the nearby causal mutation even though the gene that carries the mutation is unknown.
Genome wide genotyping using the Illumina version 1 SNP array has been performed on 366 dogs of several breeds (Zhou et al., 2010). Among these dogs, 180 Labrador Retrievers were genotyped. Genomic hip breeding values for the Norberg angle were calculated in a Bayesian framework (Guo et al., 2011). The estimated hip breeding values of these Labrador Retrievers were correlated with their genomic breeding values. The most predictive (effective) 280 SNPs were incorporated into a custom SNP array as well as 104 SNPs associated or linked to hip dysplasia from our and others previous studies. In Guo et al., (2011), we further report that the genomic hip prediction correlates between 65% and 75% to the estimated hip breeding values in German Shepherds, Rottweillers, Newfoundland dogs, Greyhounds, and Golden Retrievers.
What is the value of genomic technology and genotyping to pure breed dogs?
Current treatments for hip dysplasia rely on radiographic diagnosis yet the trait is expressed during development at 2-7 months of age when traditional imaging is inaccurate. Identification of neonatal dogs susceptible to hip dysplasia will allow earlier intervention with medical, nutritional, and surgical treatment. Once the pathobiology is understood, novel, earlier, and more successful treatment will emerge as well as specific mutation based tests. Genomic tools to help in decision making about purchasing and breeding of healthier pure breed dogs affected by one or several of these traits and diseases will be developed. Genomic data will also be used to provide inbreeding coefficients to encourage genetic diversity within each breed. Eventually, discovery of the mutations that contribute to these disorders will enable the most accurate breeding decisions based on causal mutations.
Elbow Dysplasia
We are beginning our search for markers and genes underlying elbow dysplasia. Elbow dysplasia is a common genetic disorder of many dog breeds. There are several disorders in the elbow that comprise elbow dysplasia, all involving abnormal formation of the bones of the elbow joint. The diagnosis of elbow dysplasia is made through orthopedic examination, and confirmed by x-rays or CT scan. Some cases are treatable with arthroscopic surgery, while others require complicated osteotomies, where a surgeon releases the pressure on one or more elbow components to allow the joint to conform more closely. Another treatment option is an implant that replaces the medial compartment of the elbow, where disease is most common, with a synthetic joint surface. In older dogs, elbow dysplasia is often managed medically but elbows can be totally replaced. Two view radiographs and accompanying EDTA blood samples for DNA isolation are needed for the ongoing genetic investigation.
Rupture of the Cranial Cruciate Ligament
Rupture of the cranial cruciate ligament is the most debilitating orthopedic trait affecting the hind limb of dogs and Americans spent over an estimated 1.3 billion dollars on its surgical treatment in 2005. Many large pure breed dogs like Labrador Retrievers suffer from both bilateral hip dysplasia and bilateral rupture of the cranial cruciate ligament. Up to one third of dogs admitted to US Veterinary Teaching Hospitals for treatment of hip dysplasia suffer from simultaneous cruciate ligament disease which requires treatment even before the hip osteoarthritis is addressed. It is an appalling state of affairs.
Dogs of any age or breed can tear their stifle or knee ligaments, most commonly the anterior (ACL) or cranial cruciate ligament (CCL) as we refer to it in animals. Rupturing the CCL causes varying degrees of lameness. If ruptures affect both knees or are associated with a tear of a meniscus in the knee, the injury is very debilitating. A ruptured knee ligament is diagnosed by clinical signs, palpation and x-ray, but MRI can be used in subtle cases with partial tears of the ACL. Surgery is the primary treatment. Rupture of the cranial cruciate ligament has been shown to have a heritability of 0.27 (Wilke et al., 2009) in the Newfoundland breed, which is the same approximate heritability as canine hip dysplasia in the general dog population (Hou et al., 2014). Four quantitative trait loci (QTL) have been identified through linkage mapping for cranial cruciate ligament disease in the Newfoundland breed (Wilke et al., 2009). For genetic mapping of CCL disease, we need a large group of affected (cases) dogs and a similarly large group of unaffected or control dogs of the same breed. Ruptures of the cranial cruciate ligament are diagnosed by palpation followed by stifle radiographs or by arthroscopy/arthrotomy during surgical correction. Controls for this trait are selected based on careful orthopedic examination specifically feeling for stability on stifle palpation (no cranial drawer or cranial thrust) and/or stifle radiography. We are actively seeking cases and controls for CCL mapping studies which will include an EDTA venous blood sample from which DNA can be isolated for genetic mapping studies and sequencing.
Co-investigators and Collaborators: George Lust, Nathan Dykes, Ursula Krotscheck, Adam Boyko, Jessica Hayward, Andrew Massaro, Elizabeth Parsley, Gabriela Wagner, Dorothy Ainsworth, Darryl Nydam, the oncologists and cardiologists from Cornell, and Magi Casal from the University of Pennsylvania. We are grateful to the many granting agencies, NIH, and Foundations who have supported our work for many years. Special thanks are due our friends and supporters who have been generous with their financial resources. Thanks also to Jennifer Young and Katlyn Wilcox who developed this web site.
Financial contributions to Dr. Todhunter's applied research program can be made through Ms. Carol Merkur, Department of Clinical Sciences, Cornell University 14853.


